Home >> Thermal, calorimetry
heat capacity |
||
Heat (thermal) Capacity C
By definition,
heat capacity (C) is the heat energy required to raise the temperature of a body by one degree (oC or K).
![]()
where,
ΔQ is the heat energy added to the body
Δθ is the temperature rise of the body
C is the heat capacity of the body
The units of heat capacity are Joules per degree. Since Kelvin and Celsius degrees are equivalent the units are: JK-1 or JC-1
Specific Heat Capacity c
By definition,
specific heat capacity (c) is the heat energy required to raise the temperature of unit mass by one degree (oC or K).
![]()
where,
ΔQ is the amount heat energy concerned
m is the mass of the body
Δθ is the temperature rise of the body
c is the specific heat capacity of the body
The units of specific heat capacity are Jkg-1K-1 or Jkg-1C-1
Determination of Specific Heat Capacity by experiment
These two methods concern the heating up a known mass and measuring the temperature rise for a known amount of electrical energy used.
Specific Heat Capacity of a liquid by an electrical method
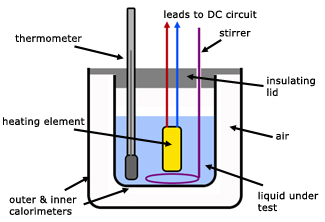
The heat energy supplied by the electrical element is given to the liquid and its container, producing a temperature rise Δθ.
The heater current (I) and voltage (V) are monitored for a time (t).
energy supplied by heater = VIt
energy absorbed by liquid and container = mLcLΔθ + mCcCΔθ
where,
mL mass of liquid
mC mass of container
cL specific heat capacity of liquid
cC specific heat capacity of container
Equating the two quantities,
![]()
mL , mC , cC are known and V, I, t, Δθ are all measured. So the specific heat capacity of the liquid (cL)can be calculated.
Specific Heat Capacity of a solid by an electrical method
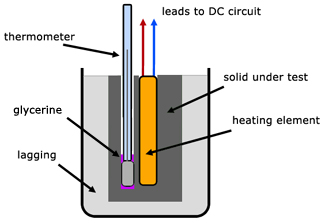
The method is very similar to that for a liquid except that there is no container. The solid under test is a lagged cylinder with holes drilled for the thermometer and the heater element. A little glycerine is added to the thermometer hole to improve thermal contact.
Heat energy supplied by the electrical element is given directly to the solid, producing a temperature rise Δθ.
![]()
where,
mS - mass of solid
cS - specific heat capacity of solid
mS is known and V, I, t, Δθ are measured. So the specific heat capacity of the solid (cS)can be calculated.
note: more accurate results can be obtained by applying a 'cooling correction'.
This is based on Newton's Law of Cooling, which states :
The rate of cooling is proportional to the excess temperature over the environment.
Unfortunately space does not allow a more in-depth treatment of this issue.
Latent Heat
Latent heat is the energy involved when a substance changes state.
Latent heat energy (L) has two components:
ΔU - the increase/decrease in internal PE
ΔW - the external work involved in expansion(+ΔW) and contraction(-ΔW)
This can be summarized as:
![]()
The phase changes involving latent heat energy are:
phase change |
action |
symbol |
solid to liquid |
melting |
LF |
liquid to solid |
fusion |
LF |
liquid to vapour |
vaporization |
LV |
vapour to liquid |
condensation |
LV |
solid to vapour |
sublimation |
LS |
vapour to solid |
sublimation |
LS |
The graph illustrates the temperature changes when a solid(eg ice) is heated from below its melting point, to above boiling.
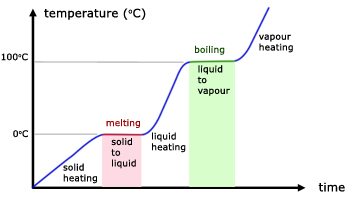
Note that the changes of state occur in the flat areas. There is no temperature rise here and hence no increase in KE.
latent heat must be absorbed from the surroundings (and given to the substance) for the substance to melt or boil.
Latent heat is given out to the surroundings (from the substance) when the substance condenses or freezes.
Specific Latent Heat Capacity l
By definition,
the latent heat of fusion of a substance is the energy involved in changing the state of unit mass of the substance at the melting/freezing point.
the latent heat of vaporization of a substance is the energy involved in changing the state of unit mass of the substance at the boiling point.
This may be summarized by the equation:
![]()
where,
ΔQ is the amount heat energy concerned
m is the mass of substance
l specific latent heat of fusion/vaporization
The units of specific heat capacity are Jkg-1.
Determination of Specific Latent heat Capacity by experiment
There are a number of different methods for finding l for different substances and different phase changes.
Here we will briefly look at two methods concerning fusion and vaporization.
The specific latent heat of ice by the 'method of mixtures'
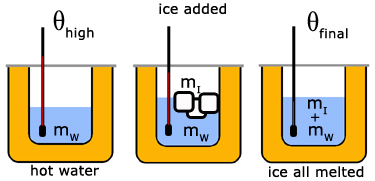
Ice cubes are added to hot water of known temperature in a copper calorimeter. The mixture is stirred until all the ice has melted and a final reading of temperature taken.

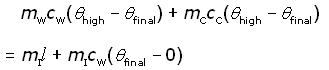
where,
mL mass of water
mI mass of ice
mC mass of calorimeter
cL specific heat capacity of liquid water
cC specific heat capacity of calorimeter
θhigh temperature of the hot water
θfinal temperature of mixture
l specific latent heat of fusion of ice
Hence l can be calculated from the knowns and measured values.
The specific latent heat of vaporization of a liquid
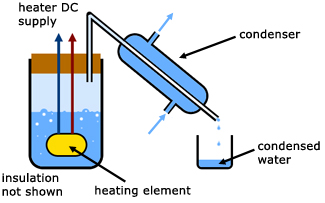
Water is heated electrically until it boils. The condensed water (m) is collected over time (t). Heating element readings of voltage (V) and current (I) are recorded.
In the steady state,
electrical energy supplied = heat energy to produce steam
![]()
[ About ] [ FAQ ] [ Links ] [ Terms & Conditions ] [ Privacy ] [ Site Map ] [ Contact ]
