Home >> Thermal, thermodynamics
zeroth law |
The Zeroth Law of Thermodynamics
This states:
If two systems are in thermal equilibrium with a third system, they are also in thermal equilibrium with each other.
To understand this concept we must first appreciate what thermal equilibrium is.
Consider a body at a high temperature in contact with a body at a low temperature. Heat is transferred from the high temperature body to the lower temperature body until the temperatures are equalized.
When this equal, constant temperature is reached and maintained, the two bodies are said to be in thermal equilibrium.
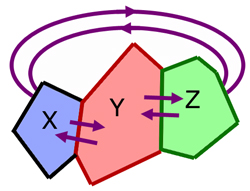
Consider three bodies, X, Y and Z.
Z is in thermal equilibrium with Y.
X is in thermal equilibrium with Y.
Then Z is in thermal equilibrium with X.
To try to visualize this further, consider a hot cup of tea.
After about twelve hours, the saucer, the cup and the tea will all be at the same temperature.
The saucer is in equilibrium with the cup.
The cup is in equilibrium with the tea.
Therefore the tea is in equilibrium with the saucer.
They are each in thermal equilibrium with eachother.
The First Law of Thermodynamics
This states:
The change in the internal energy* ( ΔU ) of a system is equal to the amount of heat supplied ( ΔQ ) to the system, minus the amount of work( ΔW ) performed by the system on its surroundings.
*Only internal energy changes can be measured. Absolute values of internal energy are not defined.
This can be stated as the equation:
![]()
note:
ΔQ is positive when heat is absorbed by the system
ΔQ is negative when heat flows away from the system
ΔW is negative when work is taken out of the system
ΔW is positive when work is put into the system
This can be better understood if we consider a mass of gas in a piston arrangement (frictionless piston, an ideal gas in the cylinder).
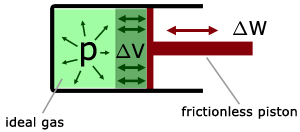
Work is done when the volume of the gas changes.
By considering dimensions it can be shown that :
work (W) = constant pressure (p) x change in volume (ΔV)
![]()
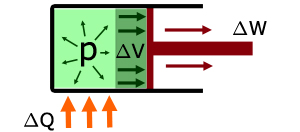
When heat energy ΔQ is supplied to the gas :
the temperature of the gas increases
work ΔW is done by the gas expanding to move the piston
the internal energy of the gas increases ΔU
the volume of the gas increases ΔV
![]()
note ΔW is negative - work is removed from the system
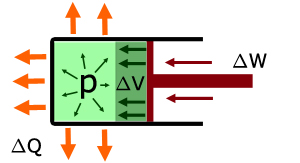
When no heat is applied and work is done externally by pushing the piston inwards to compress the gas:
the temperature of the gas increases
work ΔW is done compressing the gas
the internal energy of the gas increases ΔU
the volume of the gas decreases ΔV
![]()
note:
ΔW is positive - work is done on the system
ΔQ is negative - heat is removed from the system
The Second Law of Thermodynamics
There are a number of statements outlining this law.
The Clausius Statement
No process is possible whose sole result is the transfer of heat from a body of lower temperature to a body of higher temperature.
The Kelvin Statement
No process is possible in which the sole result is the absorption of heat from a reservoir and its complete conversion into work.
A general statement
It is not possible to convert heat continuously into work without at the same time transferring some heat from a warmer body to a colder one.
Heat engines & heat pumps
Heat engines are devices that use the flow of energy from a hot source to a cold sink to produce work.
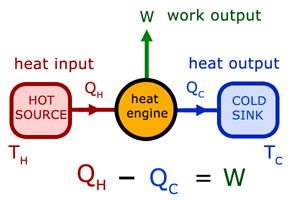
The Thermal Efficiency (η - eta) is given by:
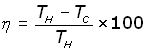
This also applies to heat pumps.
In reality the efficiency of an actual engine (eg a petrol engine) is much less than theory predicts. There are a two main reasons:
1) frictional effects
2) heat is absorbed and emitted over a range of temperatures
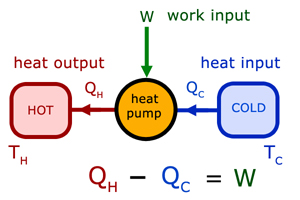
Heat pumps are devices that use work to alter the energy flow from cold to hot (eg a refridgerator).
[ About ] [ FAQ ] [ Links ] [ Terms & Conditions ] [ Privacy ] [ Site Map ] [ Contact ]
