Home >> Matter, intermolecular force & P.E.
molecules |
Molecules and atoms (considered the same)
While the kinetic theory of matter considers the motion of molecules to be free and random, there are forces between molecules.
The force acting between molecules is an electrostatic force. If we consider two molecules, the force is:
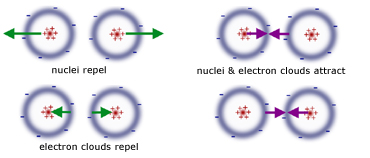
repulsive between opposite electron clouds
repulsive between opposite nuclei
attractive between one electron cloud and the opposing nucleus
As molecules have forces between them they must have potential energy.
It is convenient to think of two molecules as having zero P.E. at infinite distance, where the attractive force between them is also zero.
Work is done when two attracting molecules are separated. So their P.E. must increase when they are parted.
However, since molecules at infinite distance have zero P.E., those at intermediary distances have negative P.E. relative to this level.
Conversely, repelling molecules have positive P.E. . Repulsive forces do work to move molecules apart.
Relation between force (F) and P.E. change (δE - delta E)
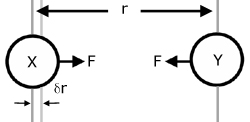
Consider two attracting molecules(X & Y). The force of attraction F of Y on X moves X a small distance δr (delta 'r') towards Y.
The distance δr is so small that the force F may be considered constant.
The work done W in moving the force F a distance δr is given by: (remember work = force x distance force moves)
![]()
If the change in the P.E. of X is δE (delta E), then:
![]()
Note the minus sign signifies the decrease in P.E. of X as it is attracted towards Y.
Elimenating δW from the first two equations:
![]()
in the limit, as δEand δr tend to zero,
![]()
This means that the negative of the gradient of an E-r graph equals the force F acting.
Curve of molecular P.E. (E) vs molecular separation (r)
The value of the equilibrium separation ro depends on the solid and temperature. An approximate value is 3 x 10-10 m.
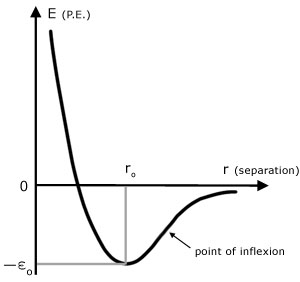
The point of inflexion is where the gradient is maximum on the right hand side of the graph.
P.E. and K.E. changes between molecules
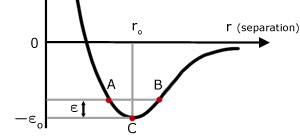
If two molecules in a solid are at absolute zero, they have no kinetic energy and their separation is ro . Now consider the two molecules at a higher temperature, with a shared kinetic energy ε . This energy creates an imbalance between the attractive and repulsive forces.
B r maximum molecules attracted PE max, KE zero
BC PE converted into KE
C r = ro equilibrium position PE min KE max
CA KE converted into PE
A r minimum molecules repelled PE max, KE zero
AC PE converted into KE
C r = ro equilibrium position PE min KE max
CB KE converted into PE
In solids molecules can only vibrate small distances about fixed positions. The reason is that the kinetic energy ε is much smaller than the potential energy εo (approx. 10%) . Hence a solid has a fixed shape and volume.
Effect of temperature on equilibrium position
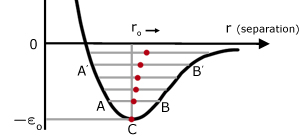
C represents the equilibrium position ro at absolute zero. In this state the molecules have no kinetic energy and consequently do not oscillate.
Consider a low temperature P.E. level at AB. The mid-point of AB moves ro to the right.
At a higher P.E. level A'B' corresponding to a higher temperature. The mid-point A'B' of moves ro even further to the right.
So as the temperature rises, ro increases. The molecules move apart and the solid is observed to expand.
Curve of intermolecular force (F) vs molecular separation (r)
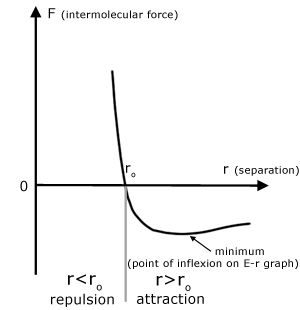
Force is the negative of the gradient of the E-r graph.
When F is positive, the force is repulsive.
When F is negative, the force is attractive.
[ About ] [ FAQ ] [ Links ] [ Terms & Conditions ] [ Privacy ] [ Site Map ] [ Contact ]
