Home >> Nuclear, radioactivity
Discovery
Radioactivity was discovered by Henri Becquerel in 1896, when he noticed 'fogging' of photographic plates that were placed in a drawer in close contact with uranium salts.
Emissions
Radioactivity is simply the spontaneous disintegration of nuclei as they move from an unstable state to a stable one.
There are three types of radiation emitted in radioactive decay: alpha particles, beta particles and gamma rays.
Alpha Particles(α)
These are helium nuclei, and therefore consist of two protons and two neutrons.
Beta Particles (β- β+)
There are two types of beta particle: beta-plus and beta-minus. The beta-plus is sometimes called an anti-electron. Each can travel up to 98% the speed of light.
A beta-minus particle is released as a result of a neutron changing into a proton, while a beta-plus particle is released as a result of a proton changing into a neutron.
Gamma Rays (γ)
Gamma rays are high energy, short wavelength photons of electromagnetic radiation. Gamma rays are emitted because the atom is usually in a high energy state after emission of alpha or beta particles. This unstable state is made stable by emission of gamma ray photons.
Balancing equations
The effect of radioactive emissions can be summarised as follows:
alpha decay:
| atomic mass A decreases by 4 (A - 4) | atomic number Z decreases by 2 (Z - 2) |
examples:
![]()
![]()
beta-minus decay:
| mass number A unchanged | atomic number Z increases by 1 (Z + 1 ) |
example:
![]()
beta-plus decay:
mass number A unchanged |
atomic number Z decreases by 1 (Z - 1 ) |
example:
![]()
(neutrinos omitted)
The Radioactive Decay Equation
The rate of decay(activity, A) is proportional to the number of parent nuclei(N) present.
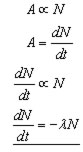
λ(lambda) is a positive constant called the decay constant. It has the unit s-1 .
The minus sign is included because N decreases as the time t in seconds (s) increases .
Half Life
The half life of a radioactive substance is the time taken for half the nuclei present to disintegrate.
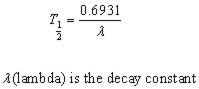
The half-life curve illustrates that that the number of nuclei halves whenever the time 't' increases by T½ . The half-life is a constant for a particular radio-nuclide.
Here is a list of half-lives of radio-nuclides from the Uraniun series.
nuclide |
|
half-life |
uranium 238 |
|
4.51 x 109 years |
thorium 234 |
|
24.1 days |
protactinium |
|
6.75 hours |
uranium 234 |
|
2.47 x 105 years |
thorium 230 |
|
8.0 x 104 years |
radium 226 |
|
1620 years |
Radioactive Equilibrium is when the rate of decay of a nuclide is approximately the same as its rate of production.
This happens to products of the Uranium Series. We start off with the production of thorium exceeding its rate of decay. Then as the amount of thorium increases, the activity increases. Eventually the rate of production of thorium equals its rate of decay. So the amount of thorium in the sample is constant.
This continues down the series with constant amounts of each product being formed in a sample. This being the case, all the rates of decay are equal.

[ About ] [ FAQ ] [ Links ] [ Terms & Conditions ] [ Privacy ] [ Site Map ] [ Contact ]
