Home >> Matter, types of solid
crystalline |
Crystalline Solids
A crystal is a regular 3D arrangement of atoms, ions or molecules. All crystals are made from identical sub units called cells.
examples of cubic cells:
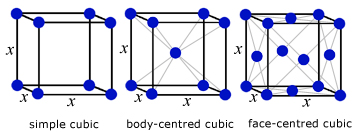
The physical properties of solids are governed to a large extent by their crystal structure(eg graphite & diamond).
It is a basic rule of physics that systems tend towards the lowest level of P.E. . Such is the case with crystals, where regular arrangements of atoms have a lower P.E. than the same atoms all jumbled up, with no structure.
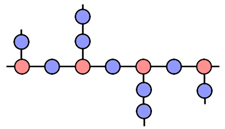
In a large single crystal similar planes of atoms are parallel to each other. So the crystal can be cleanly cut or cleaved along these planes.

This is not the case with polycrystalline solids. In this case, a solid consists of many small crystals called crystallites(or grains).
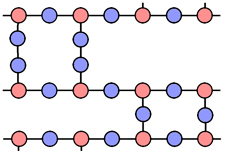
large single crystals display a property called anisotrophy. This means that physical properties(eg resistivity, linear expansion) are different for different directions along crystal axes.
Amorphous Solids
Unlike crystals, amorphous solids have no definite structure. Further, they melt not at one temperature, like crystals, but over a range of temperatures.
Examples of amorphous solids:
wax, glass, ice, toffee, polythene
Amorphous solids behave more like liquids with high viscosities. For example, over time, a vertical pane of glass is observed to be thicker at the bottom than at the top.
Glasses
Glasses are electrical insulators and transmit I.R. radiation. Manufacture is by melting mixtures of their components and cooling so that an amorphous solid is produced.
Types of glass:
Soda-Lime
Soda-lime glass is the commonest type of glass and is made from a mixture of silica SiO2, calcium oxide CaO and sodium oxide Na2O.
Its main uses are window panes and bottles.Lead Crystal
Lead crystal glass has a high refractive index and a relatively soft surface that can be easily cut. For these reasons it is used for wine glasses, decanters, flower vases, bowls etc.
In the manufacture of lead crystal, lead oxide PbO is used instead of calcium oxide CaO and potassium oxide K2O is used instead of sodium oxide Na2O.Borosilicate
Borosilicate glass is heat-resisting and has many uses in the kitchen, laboratory and in industry. It familiarly known under its trade name Pyrex.
Fused Quartz
Fused quartz is manufactured by melting pure quartz crystals at temperatures around 2000 °C. It transmits U.V. and has low expansivity. Applications include: optics(lenses & mirrors), halogen lamps, optic fibre, acid glassware, high temperature industrial use.
Polymers
Polymer are long chain molecules made from smaller units called monomers.
The simplest polymer is polythene. This is made by the process called polymerization, whereby a monomer double bond is opened out to make a polymer, with side bonds.
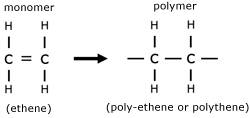
Polymer chain molecules fall into three distinct types:
Linear - have flexibility (molecules slide past one another)
Branched - increased rigidity, lower density, m.p. & strength (molecules cannot easily pack together)
Cross-Linked - very rigid ( no molecular sliding)
The bonds in polymers are mostly covalent. As a result, polymers have low thermal and electrical conductivities.
Their densities are quite low compared to metals and ceramics.
They have low melting points and can be adversely affected by sunlight.
While polymers are cheap to produce and are resistant to water and acids, they do degrade when exposed to organic solvents.
The properties of polymers can be enhanced by the addition of certain chemicals:
lead is added to PVC to stabalise it against decomposition by sunlight
mica is added to thermsetting plastics to increase electrical resistance
glass fibre is added to resins to improve strength
Thermoplastics (eg polystyrene, acrylic, PTFE, PVC)
These become pliable on slight heating, becoming rigid again on cooling.
The molecules are only weakly bound to each other. So chains are able to move with some freedom, taking up the shape of the mould.
Thermosetting plastics (eg bakelite, ebonite, epoxy resins)
Unlike thermoplastics, thermosetting plastics do not become pliable on heating. The moulding process takes place before polymerization is complete. After further heating the plastic sets, its shape becoming permanent.
As a result of the strong bonding between adjacent chains, thermosetting plastics can withstand considerable heating before decomposing.
Elastomers (eg raw & vulcanized rubber, neoprene)
Elastomers can produce very large extensions before returning to their original length, when the extending force is removed.
The elastic properties of elastomers are primarily a result of cross-links between adjacent sliding molecules. The large extensions produced by these materials is explained by tangled molecules becoming untangled in the large spaces between chains.
[ About ] [ FAQ ] [ Links ] [ Terms & Conditions ] [ Privacy ] [ Site Map ] [ Contact ]